Unit 9: Forms of Energy
Unit 9: Forms of Energy

Unit 9: Forms of Energy

Unit 9: Forms of Energy
This unit explores how energy can exist in various forms, such as mechanical, chemical, electrical, heat, sound, and nuclear. Energy can be converted from one form to another but never created or destroyed.
Student Goals
- Explain how energy can be changed from one form to another.
- Give examples of energy conversion.
- Explain chemical energy and demonstrate it through laboratory experiences.
- Describe the sources of nuclear energy, magnetic and electrical forces, and heat, and understand how the laws of thermodynamics relate to all.
- State that energy cannot be created or destroyed.
- Discuss the importance of energy to all branches of science and comprehend how energy and matter create the universe's structure.
Unit Focus
- Understand how knowledge of energy is fundamental to all the scientific disciplines (e.g., the energy required for biological processes in living organisms and the energy needed for the building, erosion, and rebuilding of the Earth).
- Understand that mass and energy are conserved when the matter is transformed.
- Describe how magnetic and electrical forces are two aspects of a single force.
- Know that the forces that hold the nucleus of an atom together are much stronger than electromagnetic forces, which is why a tremendous amount of energy is released from the nuclear reactions in the sun and other stars.
Conservation of Energy
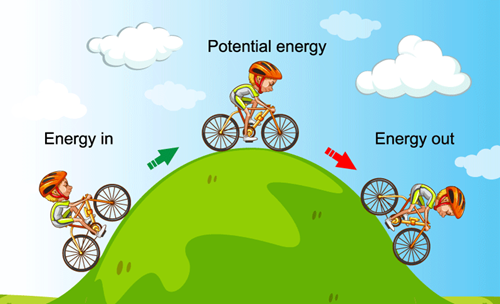
The law of conservation of energy is a fundamental principle in physics that states that the total energy of an isolated system remains constant over time. In other words, energy cannot be created or destroyed but can only change forms.
Vocabulary
Lesson Reading
Videos and Interactives (Click on Images to View Content)
