Unit 11: Nuclear Energy
Unit 11: Nuclear Energy

Unit 11: Nuclear Energy
Unit 11: Nuclear Energy
This unit explains that atoms store huge amounts of energy. Students will learn about fission and fusion and also gain knowledge about the nature of nuclear changes and their impact on living things.
Student Goals
- Develop an understanding of the nature, properties, types, and uses πof nuclear radiations.
- Describe how nuclear fission and fusion may be used for energy.
- Define chain reaction, nuclear reactor, and control rod, and describe their interaction in nuclear power plants.
- State positive and negative reasons concerning the continued development of the nuclear fission reactor.
- Define radioactivity.
- Understand the process by which scientific ideas are conceived and developed.
Unit Focus
- Know that a number of elements have heavier, unstable nuclei that decay, spontaneously giving off small particles and waves that result in a small loss of mass and release a large amount of energy.
- Know that nuclear energy is released when smaol, light atoms are fused into heavier ones.
- Understand that there is conservation of mass and energy when matter is transformed.
- Know that the forces that hold the nucleus of an atom together are much stronger than electromagnetic force and that this is the reason for the great amount of energy released from the nuclear reactions in the sun and other stars.
Radioactive Materials
Radioactive materials contain unstable atoms that undergo spontaneous nuclear transformations, emitting radiation in the process. This phenomenon is known as radioactivity. Radioactivity is a natural property of certain elements, and it plays a crucial role in various scientific, medical, and industrial applications. Here's an overview of key concepts related to radioactive materials:
Nuclear Structure:
- At the core of an atom is the nucleus, composed of protons and neutrons. The number of protons defines the element, and the total number of protons and neutrons determines the atomic mass.
- Some nuclei are unstable because they have an imbalance of protons and neutrons. This instability can lead to the spontaneous emission of particles or energy.
Types of Radioactive Decay:
- There are several types of radioactive decay, each associated with the emission of specific particles. The three main types are:
- Alpha Decay: Involves the emission of an alpha particle (two protons and two neutrons).
- Beta Decay: Involves the conversion of a neutron into a proton (beta-plus decay) or a proton into a neutron (beta-minus decay), along with the emission of a beta particle (an electron or positron).
- Gamma Decay: Involves the emission of a gamma ray, which is a high-energy electromagnetic wave.
- There are several types of radioactive decay, each associated with the emission of specific particles. The three main types are:
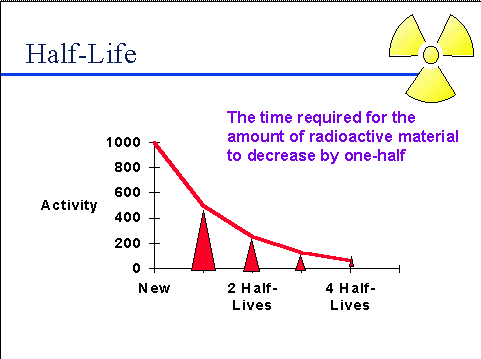 Half-Life:
Half-Life:- The half-life of a radioactive material is the time it takes for half of a sample of that material to undergo radioactive decay. It is a characteristic property of each radioactive isotope.
- The half-life provides a measure of the stability or instability of a radioactive substance. Some isotopes have very short half-lives, while others may have half-lives lasting thousands or millions of years.
Radioactive Isotopes:
- Radioactive elements exist in different isotopes, each with a unique number of neutrons. Some isotopes are stable, while others are radioactive.
- Common radioactive isotopes include uranium-235, thorium-232, radium-226, and carbon-14. Each of these isotopes decays through specific processes.
Radiation Detection:
- Geiger-Muller counters, scintillation detectors, and other devices are used to detect and measure radiation. These instruments help assess the level of radioactivity in a given material or environment.
Uses of Radioactive Materials:
- Medical Imaging: Radioactive tracers are used in procedures like positron emission tomography (PET) scans.
- Nuclear Power: Radioactive isotopes, such as uranium-235, are used as fuel in nuclear power plants to generate electricity.
- Industrial Applications: Radioactive materials are used in various industries for testing, inspection, and measurement purposes.
Radiation Safety:
- Exposure to high levels of radiation can be harmful to living organisms. Safety measures are implemented in industries working with radioactive materials, and protective gear is used to minimize exposure in medical and research settings.
Understanding radioactive materials involves knowledge of nuclear physics, decay processes, and the applications of radiation in different fields. Strict safety protocols and regulations govern the handling and disposal of radioactive substances to minimize potential risks.
Vocabulary
Lesson Reading
Videos and Interactives (Click on Images to View Content)

