Unit 2: Matter
Unit 2: Matter

Unit 2: Matter

Unit 2: Matter
This unit describes the properties of matter. The physical and chemical properties of matter are discussed. Students will learn to recognize the three phases of matter.
Student Goals
- Demonstrate, through scientific instruments, that matter occupies space and has mass.
- Differentiate between physical and chemical properties.
- Name the phases of matter and identify their characteristics.
Unit Focus
- Know that changing from one phase of matter to another involves a gain or loss of energy.
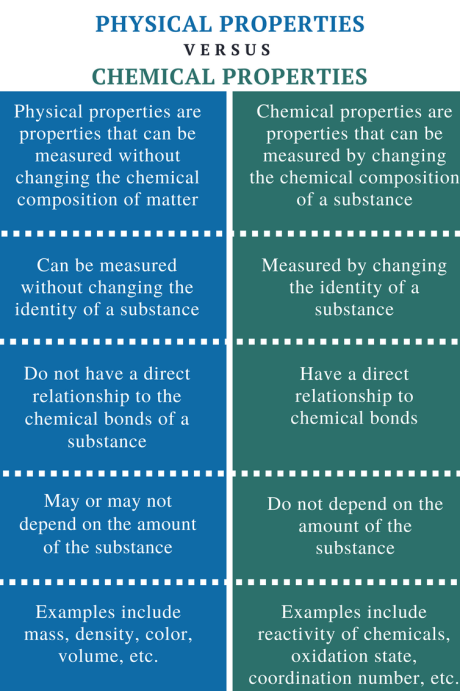
Physical Properties Versus Chemical Properties
Physical and chemical properties are categories used to describe and characterize different aspects of matter. These properties help scientists and researchers understand the behavior and nature of substances. Let's explore each category:
Physical Properties:
- Color: The visual appearance of a substance.
- Odor: The characteristic smell of a substance.
- Density: Mass per unit volume.
- Melting Point: The temperature at which a solid turns into a liquid.
- Boiling Point: The temperature at which a liquid turns into a gas.
- Solubility: The ability of a substance to dissolve in a solvent.
- Conductivity: The ability of a material to conduct heat or electricity.
- Hardness: The resistance of a material to deformation or scratching.
- Malleability: The ability of a material to deform under pressure without breaking.
- Ductility: The ability of a material to stretch without breaking.
Chemical Properties:
- Flammability: The ability of a substance to catch fire and burn.
- Reactivity: How readily a substance undergoes chemical changes with other substances.
- Corrosiveness: The ability of a substance to deteriorate or destroy other materials.
- Acidity/Basicity (pH): A substance's acidity or basicity level.
- Toxicity: The degree to which a substance can cause harm to living organisms.
- Oxidation State: The charge of an atom in a compound, indicating its ability to gain or lose electrons.
- Chemical Stability: The ability of a substance to resist chemical changes over time.
- Radioactivity: The emission of radiation by certain substances.
Differences:
Nature:
- Physical properties describe the characteristics and behavior of matter without changing its composition.
- Chemical properties describe how a substance interacts with other substances, indicating potential changes in composition.
Changes:
- Physical changes alter the state or form of a substance without changing its chemical composition.
- Chemical changes result in the formation of new substances with different chemical compositions.
Understanding physical and chemical properties is crucial for various scientific disciplines, including chemistry, physics, and materials science. These properties help predict how substances will behave under different conditions and enable the development of new materials and technologies.
Vocabulary
Lesson Reading
Videos and Interactives (Click on Images to View Content)