Unit 3: Changes in Matter
Unit 3: Changes in Matter

Unit 3: Changes in Matter
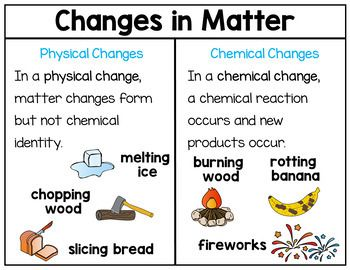
Unit 3: Changes in Matter
This unit explains physical and chemical changes in matter. Students will learn that phase changes involve energy transfer and changes in the nature of attraction between the molecules.
Student Goals
- Differentiate between physical and chemical changes through laboratory experiences.
- Know that phase changes involve energy transfer.
- Know that changes in phases are changes in the nature of the attractions between molecules.
- State that chemical changes produce new substances and energy moves and/or changes form.
- Understand that there is conservation of mass and energy when matter is changed.
Unit Focus
- Know that the vast diversity of the properties of materials is primarily due to variations in the forces that hold molecules together.
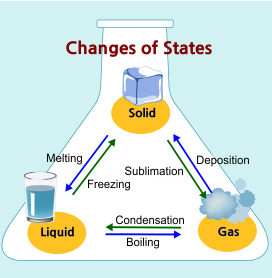
- Know that a change from one phase of matter to another involves a gain or loss of energy. (SC.A.1.4.3)
- Understand that there is conservation of mass and energy when matter is transformed.
Conservation of Mass
Vocabulary
Lesson Reading
Videos and Interactives (Click on Images to View Content)
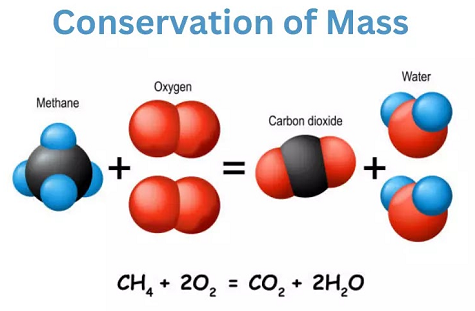 The Law of Conservation of Mass
The Law of Conservation of Mass