Unit 5: Chemical Formulas and Equations
Unit 5: Chemical Formulas and Equations

Unit 5: Chemical Formulas and Equations
Unit 5: Chemical Formulas and Equations
Chemical Formulas:
Definition:
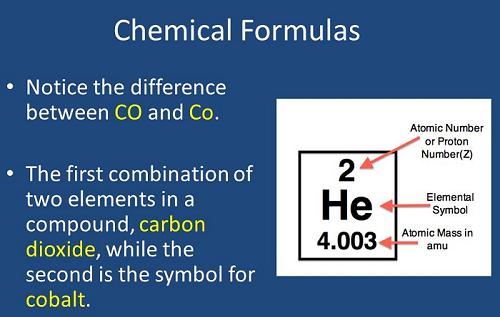
- Chemical formulas are symbolic representations of compounds, indicating the types and numbers of atoms present.
Types of Formulas:
- Molecular Formula:
- Represents the actual number of each type of atom in a molecule.
- Empirical Formula:
- Represents the simplest whole-number ratio of different atoms in a compound.
- Molecular Formula:
Representation:
- Elements are represented by symbols (e.g., for oxygen).
- Compounds are represented by combinations of symbols (e.g., for water).
Chemical Equations:
Definition:
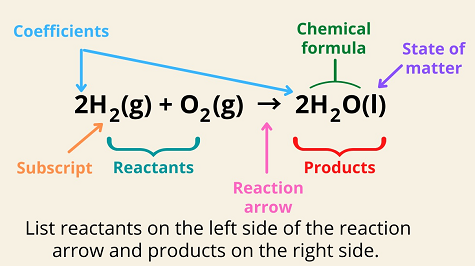
- Chemical equations are symbolic representations of chemical reactions, showing the reactants and products involved.
Basic Format:
Balancing Equations:
- Equations must be balanced to satisfy the Law of Conservation of Mass.
- Coefficients are used to balance equations, representing the number of moles of each substance.
State Symbols:
- Indicate the physical state of substances (solid, liquid, gas, aqueous solution).
Conservation of Mass:
- The total mass of the reactants equals the total mass of the products.
Limiting Reactant:
- The limiting reactant is the substance that is completely consumed in a reaction.
Yields:
- The arrow in a chemical equation indicates the direction of the reaction.
Student Goals
- Recognize the difference between a chemical formula and a chemical equation.
- Identify a simple balanced chemical equation.
- Identify chemical equations that are examples of the law of conservation of mass.
- Cite evidence, determined from an experiment using chemical equations, to support the law of conservation of mass.
Unit Focus
- Know the difference between an element, a molecule, and a compound.
- Understand that there is conservation of mass and energy when matter is transformed.
Vocabulary
Lesson Reading
Videos and Interactives (Click on Images to View Content)