Unit 7: The Periodic Table
This unit introduces the periodic table and how the table is organized. Students will learn about atomic structure and how to use the periodic table.
The periodic table is a systematic arrangement of chemical elements based on their atomic number, electron configuration, and chemical properties. Here's an overview of its key features:
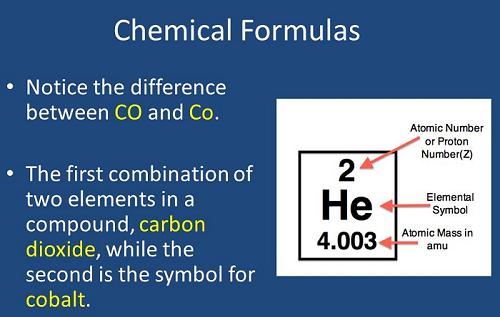
Atomic Number: Elements are ordered by increasing atomic number, which represents the number of protons in an atom's nucleus. The atomic number uniquely identifies each element.
Rows and Columns: The table is organized into rows called periods and columns called groups. Elements in the same group have similar chemical properties, while those in the same period share the same energy levels.
Groups: There are 18 groups in the periodic table, each denoted by a number or letter. Common groups include alkali metals (Group 1), alkaline earth metals (Group 2), halogens (Group 17), and noble gases (Group 18).
Blocks: Elements are categorized into blocks based on the subshell in which the last electron is added. These include s-block, p-block, d-block, and f-block.
Metals, Non-metals, Metalloids: Elements are classified as metals, non-metals, or metalloids based on their physical and chemical properties. Metals generally have luster, conductivity, and malleability, while non-metals may be gases or brittle solids. Metalloids exhibit properties of both.
Periodic Trends: Certain properties of elements exhibit periodic trends. For example, atomic radius generally decreases across a period and increases down a group.
 Transition Metals: Transition metals are found in the d-block and are known for their variable oxidation states and diverse chemical behaviors.
Transition Metals: Transition metals are found in the d-block and are known for their variable oxidation states and diverse chemical behaviors.
Noble Gases: Group 18 elements are noble gases, which are typically inert and have full electron shells.
Valence Electrons: The group number often corresponds to the number of valence electrons an element has. Valence electrons are the electrons in the outermost energy level and play a crucial role in chemical bonding.
Mendeleev's Periodic Law: The modern periodic table builds upon Dmitri Mendeleev's early work, where he organized elements based on their atomic masses and predicted the properties of undiscovered elements.
The periodic table is a fundamental tool in chemistry, aiding scientists in understanding the relationships between elements and predicting their behavior. It provides a structured framework for organizing the vast array of chemical elements and their properties.
Student Goals
- Understand the organization of the periodic table by groups and periods.
- Identify certain elements by their symbols.
- Find information necessary to construct a diagram showing the atomic structure of an element.
- Determine the atomic masses and numbers of certain elements when given the essential data.
- Understand how theories in science develop and evolve, how theories are accepted or rejected, and how theories are based on certain assumptions.
Unit Focus
- Know that from time to time, major shifts occur in the scientific view of how the world works, but that more often, the changes that take place in the body of scientific knowledge are small modifications of prior knowledge.
- Understand that no matter how well one theory fits observations, a new theory might fit them as well or better, or might fit a wider range of observations, because in science, the testing, revising, and occasional discarding of theories, new and old, never ends and leads to an increasingly better understanding of how things work in the world, but not to absolute truth.
- Know that the number and configuration of electrons will equal the number of protons in an electrically neutral atom and when an atom gains or loses electrons, the charge is unbalanced. (SC.A.2.4.1)
- Know that elements are arranged into groups and families based on similarities in electron structure and that their physical and chemical properties can be predicted.


 Transition Metals:
Transition Metals: