Unit 8: Chemical Reactions
Unit 8: Chemical Reactions

Unit 8: Chemical Reactions
Unit 8: Chemical Reactions
This unit emphasizes the factors that control and affect chemical reactions. Students will learn to represent the configurations of electrons for atoms and compounds and learn how these configurations determine what reactions are possible. Students are introduced to some concepts in biochemistry and are shown how biochemical reactions follow other chemical laws.
Chemical reactions involve the transformation of one or more substances into new substances with different chemical properties. Here are key points about chemical reactions:
1. Chemical Equations:
- Chemical reactions are represented by chemical equations.
- The reactants (starting substances) are written on the left, and the products (resulting substances) are written on the right.
2. Balancing Equations:
- Equations must be balanced to satisfy the Law of Conservation of Mass.
- The number of atoms of each element on the reactant side must equal the number on the product side.
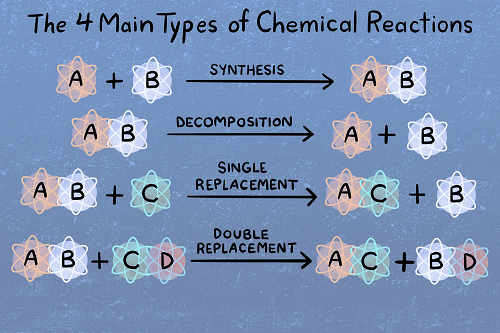 3. Reactants and Products:
3. Reactants and Products:
- Reactants undergo a chemical change to form products.
- The arrow (→) indicates the direction of the reaction.
4. Types of Reactions:
- Combination (Synthesis):
- Decomposition:
- Single Replacement (Displacement):
- Double Replacement (Metathesis):
5. Redox Reactions:
- Involve the transfer of electrons.
- Oxidation is the loss of electrons, and reduction is the gain of electrons.
6. Exothermic and Endothermic Reactions:
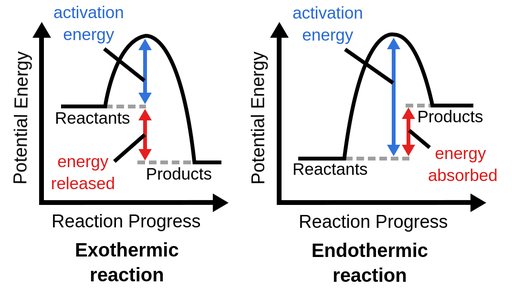 Exothermic:
Exothermic:- Release heat to the surroundings.
- Often feel warm or hot.
- Endothermic:
- Absorb heat from the surroundings.
- Often feel cool or cold.
7. Reaction Rates:
- The speed at which a reaction occurs.
- Influenced by temperature, concentration, surface area, and catalysts.
8. Limiting Reactant:
- The reactant that is completely consumed, limiting the amount of product formed.
9. Catalysts:
- Substances that speed up the rate of a reaction without being consumed.
- Provide an alternative reaction pathway with lower activation energy.
10.  Chemical Energy:
Chemical Energy:
- Chemical reactions involve the conversion of chemical energy.
- Energy is either absorbed or released during a reaction.
11. Equilibrium:
- Some reactions reach a state of equilibrium, where the rates of the forward and reverse reactions are equal.
- Denoted by a double arrow ().
12. Acids and Bases:
- Acid-base reactions involve the transfer of protons (H⁺ ions).
Understanding chemical reactions is essential in chemistry, providing insights into the composition, properties, and behavior of substances. It forms the basis for various applications, including industrial processes, environmental chemistry, and the synthesis of new materials.
The Role of Electrons
The role of electrons in chemistry is central to understanding the behavior of atoms and the formation of chemical compounds. Here are key aspects of the role of electrons:
1. Atomic Structure:
- Electrons are subatomic particles with a negative charge.
- Orbit the nucleus in specific energy levels or electron shells.
- The arrangement of electrons in these shells determines the chemical properties of an element.
2. Chemical Bonding:
- Atoms bond to achieve a more stable electron configuration.
- Covalent Bonding:
- Involves the sharing of electrons between atoms.
- Forms molecules, such as H₂O (water) or CH₄ (methane).
- Ionic Bonding:
- Involves the transfer of electrons from one atom to another.
- Forms ions with positive and negative charges that attract each other.
3. Valence Electrons:
- Electrons in the outermost shell are called valence electrons.
- Determine the chemical reactivity and bonding capabilities of an atom.
- Elements in the same group (column) of the periodic table have similar valence electron configurations.
4. Chemical Reactions:
Chemical Reactions:
- Chemical reactions involve the rearrangement of electrons.
- Electrons play a key role in the breaking and forming of chemical bonds during reactions.
5. Redox Reactions:
- Involves the transfer of electrons between reactants.
- Oxidation is the loss of electrons, and reduction is the gain of electrons.
6. Free Radicals:
- Unpaired electrons can lead to the formation of free radicals.
- Free radicals can be highly reactive and participate in various chemical reactions.
7. Electron Configuration:
- Describes the distribution of electrons in an atom's electron shells.
- Follows specific rules, such as the Pauli Exclusion Principle and Hund's Rule.
8. Energy Levels:
- Electrons in higher energy levels have higher energy.
- Absorption or release of energy occurs when electrons move between energy levels.
9. Quantum Mechanics:
- Describes the behavior of electrons using principles like wave-particle duality and Heisenberg's uncertainty principle.
10. Electron Cloud:
- Describes the probabilistic distribution of electrons around the nucleus.
- Electrons do not follow fixed paths but exist within a cloud of probability.
Understanding the role of electrons is crucial for explaining chemical bonding, predicting reactivity, and interpreting the behavior of atoms and molecules in various chemical processes. The principles of quantum mechanics and the behavior of electrons contribute significantly to our understanding of the microscopic world of chemistry.
Vocabulary
Lesson Reading
Videos and Interactives (Click on Images to View Content)
