Unit 9: Solutions and Suspensions
Unit 9: Solutions and Suspensions

Unit 9: Solutions and Suspensions
Unit 9: Solutions and Suspensions
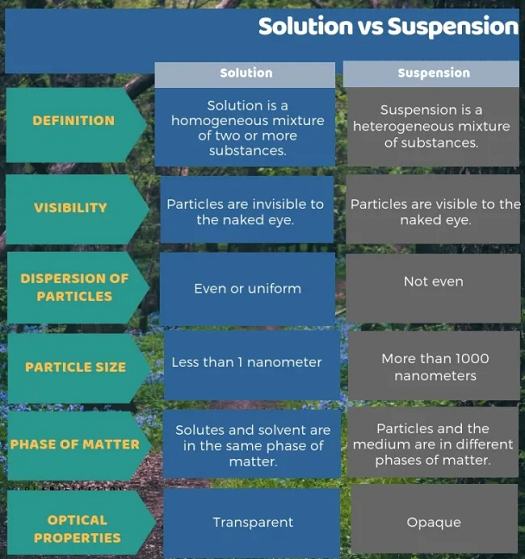
A solution is always transparent; light passes through with no scattering from solute particles, which are molecules in size. The solution is homogeneous and does not settle out. A solution cannot be filtered but separated using the distillation process.
A suspension is cloudy and heterogeneous. The particles are more significant than 10,000 Angstroms, which allows them to be filtered. If a suspension is allowed to stand, the particles will separate.
Suspension vs Solution
Chemistry is the physical science that deals with matter and the changes it goes through during chemical reactions. It deals with the chemical reaction between mixed substances and how they are transformed into another substance.
- Solutions and suspensions are mixtures of different substances. They are formed by combining a substance with one or more substances that have different characteristics.
- Solutions are homogeneous; their volumes have uniform components and properties. The sizes of the particles in solutions are at the ion or molecular level. They are transparent, and light can pass through them.
- Solutions have two components: the solute, the material to be dissolved, and the solvent, which is the substance that dissolves the solute. The solution can have color if the solute can absorb light. In a solution, the solvent completely dissolves the solute and goes through a chemical change.
Solvents can be:
- Gases that can dissolve other forms of gas. An example is air, which is oxygen dissolved in nitrogen.
- Liquids which can dissolve gases, solids, and liquids. An example is carbonated water, which is carbon dioxide dissolved in water.
- Solids which can dissolve solids, liquids, and gases. An example is steel, wherein carbon atoms are dissolved in iron atoms.
The components of a solution cannot be separated by filtration or by letting it stand. Solubility can either be miscible, wherein two liquids completely dissolve, or immiscible, wherein two substances cannot form a solution when mixed. An example of immiscibility is water and oil.
On the other hand, suspensions are heterogeneous, with volumes having different properties. The particles of suspensions are large and can be seen by the naked eye. They are opaque and murky, and light cannot pass through them. Suspensions are unstable, and their components separate on standing. They can be separated by filtration and are classified according to their dispersed phase, solid, and their dispersion medium, solid, liquid, or gas.
Examples of suspensions are flour, chalk powder, and soil, suspended in water (mud), blood, paint, dust suspended in the air, aerosol spray, algae in water, and sand in water.
Vocabulary
Lesson Reading
Videos and Interactives (Click on Images to View Content)