Unit 6: Atomic Theory
Unit 6: Atomic Theory

Unit 6: Atomic Theory
Unit 6: Atomic Theory
An atomic theory is a model developed to explain the properties and behaviors of atoms. As with any scientific theory, an atomic theory is based on scientific evidence available at any given time and serves to suggest future lines of research about atoms.
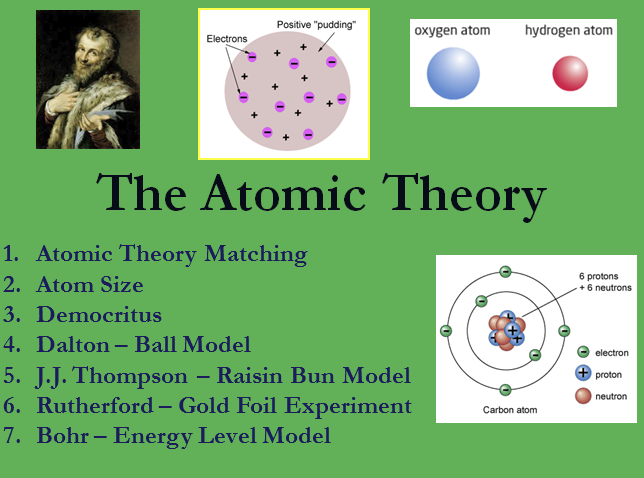
The concept of an atom can be traced to debates among Greek philosophers that took place around the sixth century B.C. One of the questions that interested these thinkers was the nature of matter. Is matter, they asked, continuous or discontinuous? That is, if you could break apart a piece of chalk as long as you wanted, would you ever reach some ultimate particle beyond which further division was impossible? Or could you keep up that process of division forever? A proponent of the ultimate particle concept was the philosopher Democritus (c. 470–c. 380 B.C. ), who named those particles atomos. In Greek, atomos means 'indivisible.'
Although the idea of the atom was first suggested by Democritus in the fourth century BC, his suppositions were not useful in explaining chemical phenomena, because there was no experimental evidence to support them. It was not until the late 1700's that early chemists began to explain chemical behavior in terms of the atom. Joseph Priestly, Antoine Lavoisier, and others set the stage for the foundation of chemistry. They demonstrated that substances could combine to form new materials. It was the English chemist, John Dalton, who put the pieces of the puzzle together and developed an atomic theory in 1803.
Dalton's atomic theory contains five basic assumptions:
- All matter consists of tiny particles called atoms. Dalton and others imagined the atoms that composed all matter as tiny, solid spheres in various stages of motion.
- Atoms are indestructible and unchangeable. Atoms of an element cannot be created, destroyed, divided into smaller pieces, or transformed into atoms of another element. Dalton based this hypothesis on the law of conservation of mass as stated by Antoine Lavoisier and others around 1785.
- Elements are characterized by the weight of their atoms. Dalton suggested that all atoms of the same element have identical weights. Therefore, every single atom of an element such as oxygen is identical to every other oxygen atom. However, atoms of different elements, such as oxygen and mercury, are different from each other.
- In chemical reactions, atoms combine in small, whole-number ratios. Experiments that Dalton and others performed indicated that chemical reactions proceed according to atom to atom ratios which were precise and well-defined.
- When elements react, their atoms may combine in more than one whole-number ratio. Dalton used this assumption to explain why the ratios of two elements in various compounds, such as oxygen and nitrogen in nitrogen oxides, differed by multiples of each other.
John Dalton's atomic theory was generally accepted because it explained the laws of conservation of mass, definite proportions, multiple proportions, and other observations. Although exceptions to Dalton's theory are now known, his theory has endured reasonably well, with modifications, throughout the years.
Vocabulary
Lesson Reading