Unit 7: Structure of Matter
Unit 7: Structure of Matter

Unit 7: Structure of Matter
Unit 7: Structure of Matter
Structure of Matter
Atoms are made from a nucleus of protons and neutrons and a cloud of electrons. Electrons are in constant motion around the nucleus, while the protons and neutrons move within the nucleus. Neutrons and protons are each composed of three quarks. As far as scientists can tell at the moment, quarks and electrons are among the most fundamental forms of matter.
The Atom
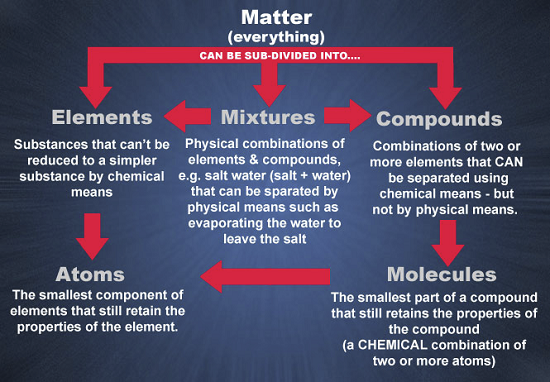
All matter such as solids, liquids, and gases, is composed of atoms. Therefore, the atom is considered to be the basic building block of matter. However, atoms are almost always grouped together with other atoms to form what is called a molecule. Only a few gases such as helium are composed of individual atoms as the structural unit.
Atoms are extremely small. The radius of a typical atom is on the order of 0.00000000001 meter and cannot be studied without very powerful microscopes. In the image below you can see how a scanning electron microscope can be used to magnify things until very small details appear relatively big.
Atoms consist of electrons surrounding a nucleus that contains protons and neutrons.
Neutrons are neutral, but protons and electrons are electrically charged. Protons have a relative charge of +1, while electrons have a relative charge of -1.
The number of protons in an atom is called its atomic number. In the periodic table atoms are arranged in atomic number order.
Electrons are arranged in energy levels or shells, and different energy levels can hold different numbers of electrons. The electronic structure of an atom is a description of how the electrons are arranged, which can be shown in a diagram or by numbers. There is a link between the position of an element in the periodic table and its electronic structure.
The structure of an atom
Although the word 'atom' comes from the Greek for indivisible, we now know that atoms are not the smallest particles of matter. Atoms are made from smaller subatomic particles.
Both protons and electrons have an electrical charge. Both have the same size of electrical charge, but the proton is positive and the electron negative. The neutron is neutral.
Vocabulary
Lesson Reading